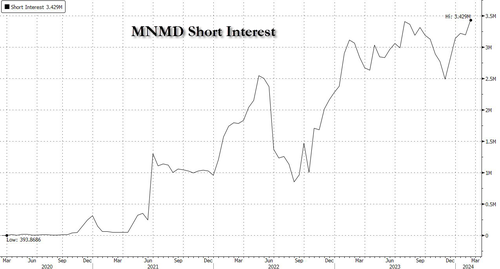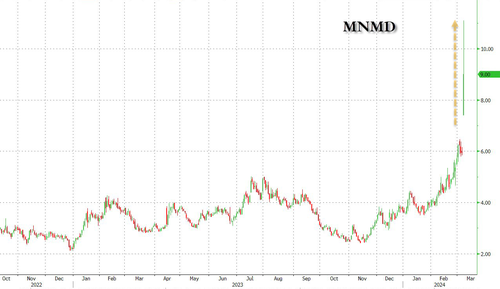
Shares of clinical stage biopharmaceutical company MindMed will once again be on watch Friday after rocketing higher during Thursday's cash session by more than 51% on more than 30x the company's average volume.
The company announced on Thursday it had received breakthrough therapy designation by the FDA and announced positive 12 week durability data from a Phase 2B study of its candidate, MM120, for generalized anxiety disorder.
The news was enough to send shares, which have an all time high short interest, rocketing higher.
David Feifel, MD, PhD, Professor Emeritus of Psychiatry at the University of California, San Diego and Director of the Kadima Neuropsychiatry Institute in La Jolla, California and an investigator in the MM120 study commented: “I’ve conducted clinical research studies in psychiatry for over two decades and have seen studies of many drugs under development for the treatment of anxiety. That MM120 exhibited rapid and robust efficacy, solidly sustained for 12 weeks after a single dose, is truly remarkable."
He continued: “These results suggest the potential MM120 has in the treatment of anxiety, and those of us who struggle every day to alleviate anxiety in our patients look forward to seeing results from future Phase 3 trials.”
Robert Barrow, Chief Executive Officer and Director of MindMed added: “The FDA’s decision to designate MM120 as a breakthrough therapy for GAD and the durability data from our Phase 2b study provide further validation of the important potential role this treatment can play in addressing the huge unmet need among individuals living with GAD.”
“We are committed to bringing MM120 to people living with GAD and delivering on the potential of our pipeline to treat serious brain health disorders,” he said.
The company said of its Phase 2B trial:
In the Phase 2b study, known as MMED008, MM120 was generally well-tolerated with most adverse events rated as mild to moderate, transient and occurring on dosing day, and being consistent with expected acute effects of the study drug. The most common adverse events (at least 10% incidence in the high dose groups) on dosing day included illusion, hallucinations, euphoric mood, anxiety, abnormal thinking, headache, paresthesia, dizziness, tremor, nausea, vomiting, feeling abnormal, mydriasis and hyperhidrosis.
Prior to treatment with MM120, study participants were clinically tapered and then washed out from any anxiolytic or antidepressant treatments and did not receive any form of study-related psychotherapy for the duration of their participation in the study.
The 57 person firm is working on using psychedelics to help treat mental health. "Rather than ameliorating distressing symptoms, MindMed is developing psychedelic inspired medicines that seek to treat underlying causes of distress in the brain," its website says.
"Serotonergic psychedelics such as LSD, psilocybin, and DMT increase the creation of new neurons and parts of neurons (neurogenesis and spinogenesis) and connections between neurons (synaptogenesis), which facilitates increased communication between neurons," MindMed's site says.
"Evidence suggests that while under the influence of psychedelics, the brain shows increased connectivity, including connections between areas of the brain that aren’t normally directly connected. This process may help to reverse some degree of the changes that result from negative experiences, depression, anxiety, PTSD, and substance misuse."
Shares of clinical stage biopharmaceutical company MindMed will once again be on watch Friday after rocketing higher during Thursday’s cash session by more than 51% on more than 30x the company’s average volume.
The company announced on Thursday it had received breakthrough therapy designation by the FDA and announced positive 12 week durability data from a Phase 2B study of its candidate, MM120, for generalized anxiety disorder.
The news was enough to send shares, which have an all time high short interest, rocketing higher.
David Feifel, MD, PhD, Professor Emeritus of Psychiatry at the University of California, San Diego and Director of the Kadima Neuropsychiatry Institute in La Jolla, California and an investigator in the MM120 study commented: “I’ve conducted clinical research studies in psychiatry for over two decades and have seen studies of many drugs under development for the treatment of anxiety. That MM120 exhibited rapid and robust efficacy, solidly sustained for 12 weeks after a single dose, is truly remarkable.”
He continued: “These results suggest the potential MM120 has in the treatment of anxiety, and those of us who struggle every day to alleviate anxiety in our patients look forward to seeing results from future Phase 3 trials.”
Robert Barrow, Chief Executive Officer and Director of MindMed added: “The FDA’s decision to designate MM120 as a breakthrough therapy for GAD and the durability data from our Phase 2b study provide further validation of the important potential role this treatment can play in addressing the huge unmet need among individuals living with GAD.”
“We are committed to bringing MM120 to people living with GAD and delivering on the potential of our pipeline to treat serious brain health disorders,” he said.
The company said of its Phase 2B trial:
In the Phase 2b study, known as MMED008, MM120 was generally well-tolerated with most adverse events rated as mild to moderate, transient and occurring on dosing day, and being consistent with expected acute effects of the study drug. The most common adverse events (at least 10% incidence in the high dose groups) on dosing day included illusion, hallucinations, euphoric mood, anxiety, abnormal thinking, headache, paresthesia, dizziness, tremor, nausea, vomiting, feeling abnormal, mydriasis and hyperhidrosis.
Prior to treatment with MM120, study participants were clinically tapered and then washed out from any anxiolytic or antidepressant treatments and did not receive any form of study-related psychotherapy for the duration of their participation in the study.
The 57 person firm is working on using psychedelics to help treat mental health. “Rather than ameliorating distressing symptoms, MindMed is developing psychedelic inspired medicines that seek to treat underlying causes of distress in the brain,” its website says.
“Serotonergic psychedelics such as LSD, psilocybin, and DMT increase the creation of new neurons and parts of neurons (neurogenesis and spinogenesis) and connections between neurons (synaptogenesis), which facilitates increased communication between neurons,” MindMed’s site says.
“Evidence suggests that while under the influence of psychedelics, the brain shows increased connectivity, including connections between areas of the brain that aren’t normally directly connected. This process may help to reverse some degree of the changes that result from negative experiences, depression, anxiety, PTSD, and substance misuse.”
Loading…