The US Food and Drug Administration has warned wholesalers, retail pharmacies, health care practitioners, and patients about counterfeit versions of Novo Nordisk's diabetes drug Ozempic. The drug has been adapted into a popular weight loss drug, taking Western nations by storm.
Bloomberg reports the FDA seized thousands of Ozempic units that were found in the nation's drug supply chain. It warned wholesalers and doctors against distributing or selling Ozempic with lot number NAR0074 and serial number 430834149057 on the packaging.
Danish drugmaker Novo Nordisk and the FDA are testing the counterfeit Ozempic to analyze its contents.
"FDA and Novo Nordisk (manufacturer of Ozempic) are testing the seized products and do not yet have information about the drugs' identity, quality, or safety," the FDA warned.
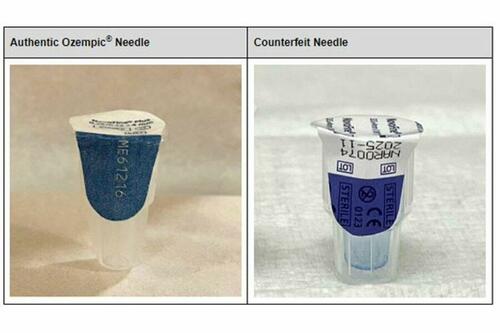
Novo pointed out that counterfeit Ozempic was found in warehouses outside its authorized supply chain. Other counterfeit components were needles, pen labels, cartons, and patient information.
"The sterility of the needles cannot be confirmed, which presents an increased risk of infection for patients who use the counterfeit products," the FDA stated.
A demand-driven shortage of Ozempic and Wegovy is overwhelming Novo's supply chain for drugs across North America and Europe as criminals take advantage of the hype cycle with counterfeits.
The US Food and Drug Administration has warned wholesalers, retail pharmacies, health care practitioners, and patients about counterfeit versions of Novo Nordisk’s diabetes drug Ozempic. The drug has been adapted into a popular weight loss drug, taking Western nations by storm.
Bloomberg reports the FDA seized thousands of Ozempic units that were found in the nation’s drug supply chain. It warned wholesalers and doctors against distributing or selling Ozempic with lot number NAR0074 and serial number 430834149057 on the packaging.
Danish drugmaker Novo Nordisk and the FDA are testing the counterfeit Ozempic to analyze its contents.
“FDA and Novo Nordisk (manufacturer of Ozempic) are testing the seized products and do not yet have information about the drugs’ identity, quality, or safety,” the FDA warned.
Novo pointed out that counterfeit Ozempic was found in warehouses outside its authorized supply chain. Other counterfeit components were needles, pen labels, cartons, and patient information.
“The sterility of the needles cannot be confirmed, which presents an increased risk of infection for patients who use the counterfeit products,” the FDA stated.
A demand-driven shortage of Ozempic and Wegovy is overwhelming Novo’s supply chain for drugs across North America and Europe as criminals take advantage of the hype cycle with counterfeits.
Loading…