Public health authorities are investigating an outbreak of bacterial infections believed to be associated with “artificial tears” eye drop usage.
The Centers for Disease Control and Prevention (CDC) announced it is working with the Food and Drug Administration (FDA) and other state and local health authorities to investigate a strain of Pseudomonas aeruginosa that the agency says is “extensively drug-resistant.”
The CDC wrote that the strain, called VIM-GES-CRPA, had not previously been seen in the United States but has now been identified in 68 patients across 16 states, with 37 cases linked to four healthcare facility clusters.
Of these cases, three deaths have been reported. In multiple cases, vision loss has been observed, and the surgical removal of a patient’s eyeball has been necessary.

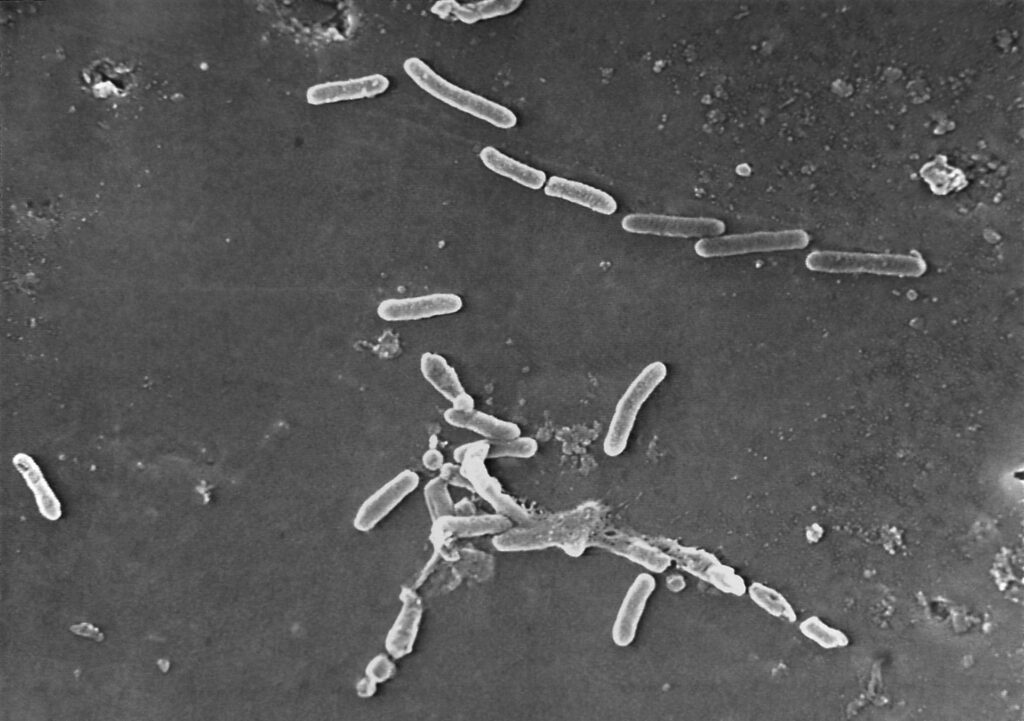
This scanning electron microscope image made available by the CDC shows rod-shaped Pseudomonas aeruginosa bacteria. U.S. health officials are advising people to stop using the over-the-counter eye drops, EzriCare Artificial Tears, that have been linked to an outbreak of drug-resistant infections. (Janice Haney Carr/CDC via AP)
EzriCare or Delsam Pharma’s Artificial Tears have been identified as a common exposure, and the CDC advised consumers and physicians to discontinue the use of these products for now. However, the agency noted that over ten other brands have been associated with the cases it has found.
NBC News reported the manufacturer of the EziCare brand product has issued a recall, admitting in its recall announcement, “The product was distributed nationwide in the USA over the internet.”
The agency wrote the contaminant has been detected in opened EzriCare Artificial Tears bottles, and tests on unopened bottles of the product are currently underway.
The CDC recommends consumers who have experienced “Yellow, green, or clear discharge from the eye,” “Eye pain or discomfort,” “Redness of the eye or eyelid,” “Feeling of something in your eye (foreign body sensation),” “Increased sensitivity to light,” or “Blurry vision” to seek medical attention.
The agency has not issued a recommendation for those who have used EzriCare or Delsam Pharma’s Artificial Tears to seek testing unless they have experienced these symptoms.
However, it has advised patients who were previously recommended these products to ask their physicians for alternatives.





