Call it wishful thinking or strategic amnesia, but just two years removed from its controversial decision ending a constitutional right to abortion, the Supreme Court is poised to decide another high-stakes appeal over nationwide access to the procedure.
At issue is the federal government’s approval process of the drug mifepristone, a medication used to terminate pregnancies.
Oral arguments are scheduled for Tuesday with a ruling expected about three months later, with the race for the next president in full swing.
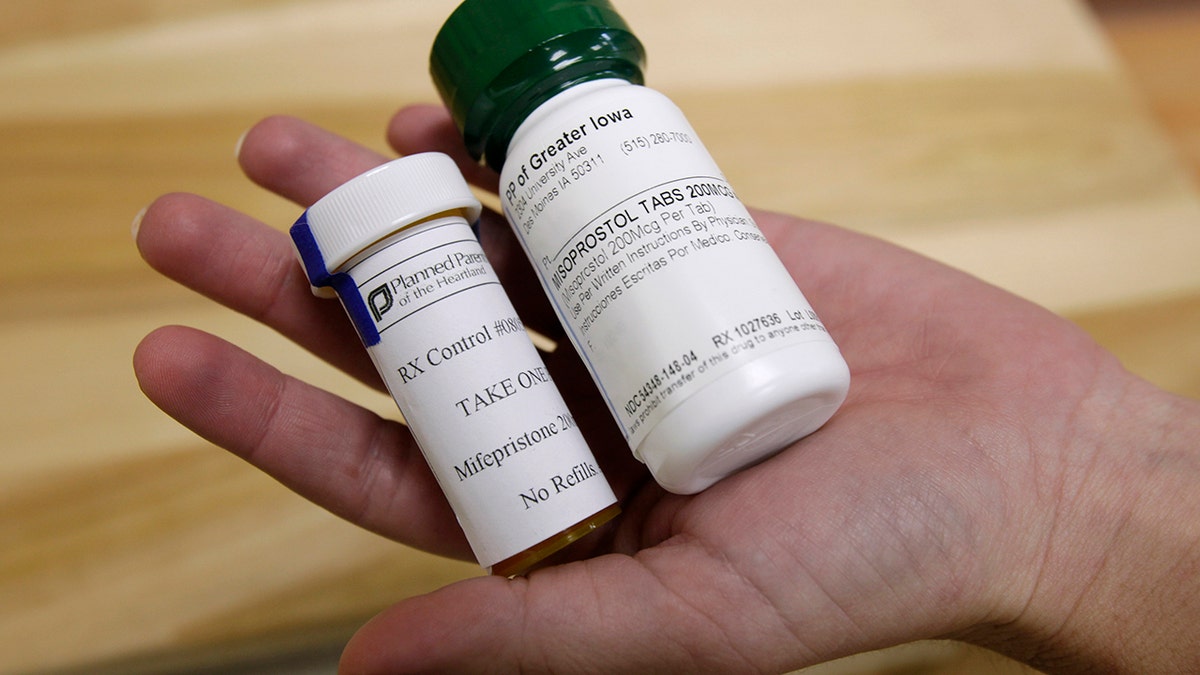
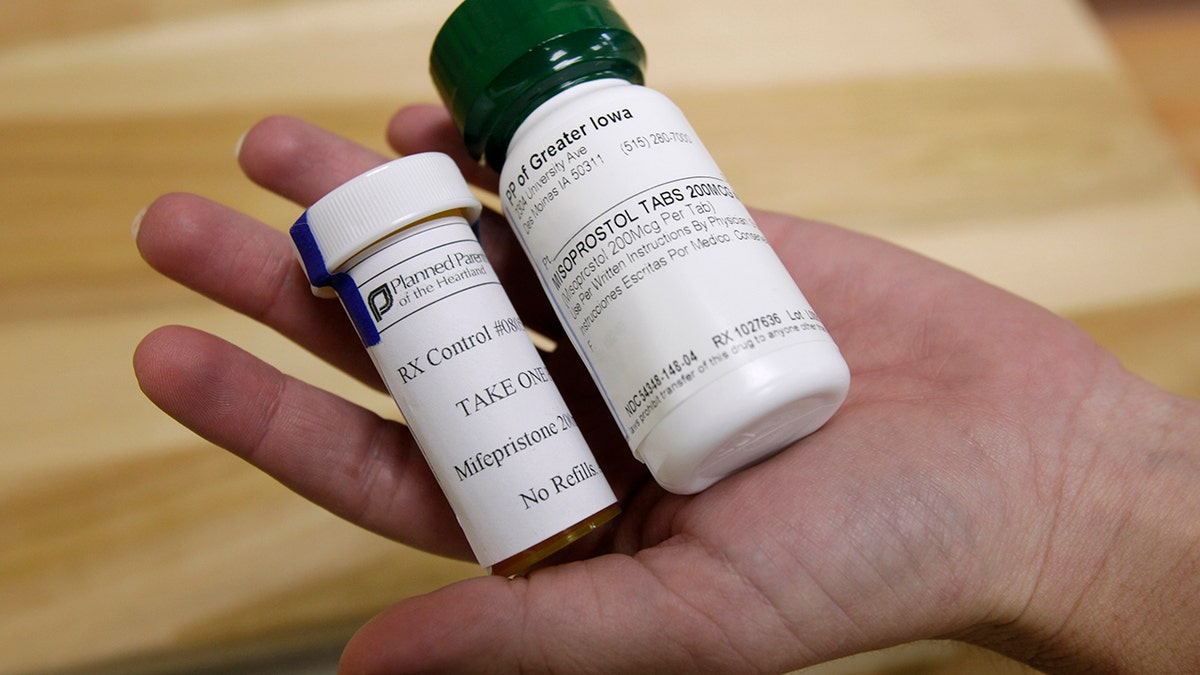
Bottles of the abortion pills mifepristone, left, and misoprostol, right, at a clinic. (Charlie Neibergall)
While the case hinges on complex federal regulatory procedures, reproductive rights will again be the key question. It’s an appeal with enormous legal, social and political implications, and a high-stakes case following the court’s landmark ruling that struck down Roe v. Wade.
NATIONWIDE IMPACT
New data from the Guttmacher Institute research group indicates nearly two-thirds of all abortions in the U.S. in 2023 rely on mifepristone. Abortion rights groups say the drug has been proven safe and that the court’s decision could negatively impact 40 million women nationwide. Anti-abortion organizations counter that the U.S. Food and Drug Administration for two decades has unlawfully promoted a nationwide regime of on-demand abortion, in defiance of several state health and safety laws.
For now, the Supreme Court is allowing the FDA to continue regulating the drug while the appeals process plays out. That includes continued telemedicine prescriptions and retail pharmacy dispensing.
FORMER SUPREME COURT JUSTICE STEPHEN BREYER SOUNDS OFF ON DOBBS DECISION: ‘TOO MANY QUESTIONS’
“I think it probably was a little premature to say that the court was going to be getting out of the abortion business entirely,” Thomas Dupree, a former top official in the justice department of former President George W. Bush. “I think the justices are aware of the fact, obviously, that we’re in an election year, but I don’t think the fact that we’re in an election year is going to be driving the outcomes of any of these decisions.”

Members of the Supreme Court (L-R) associate justices Amy Coney Barrett, Neil M. Gorsuch, Sonia Sotomayor and Clarence Thomas; Chief Justice John G. Roberts, Jr.; and associate justices Ketanji Brown Jackson, Samuel A. Alito, Jr., Elena Kagan and Brett M. Kavanaugh. (Collection of the Supreme Court of the United States)
The issues presented come nearly two years after the Supreme Court overturned the nationwide, constitutional right to abortion, giving states individual discretion to regulate the procedure.
At the time, the court’s 5-4 conservative majority declared “unelected members of this Court” would not be intervening in the future to “override the democratic process” of legislators and mandate national abortion policy.
APPROVAL PROCESS
But recent legal challenges from anti-abortion groups questioned the FDA’s original 2000 nationwide approval process – including recent revisions – for the drug used to terminate early pregnancies.
Mifepristone is taken along with misoprostol, and the two-drug combination is known as medication abortion or the “abortion pill.” Lower courts concluded the federal agency did not fully consider the potential health risks to women when revising regulations for mifepristone beginning in 2016.
Those revisions — last updated in 2023 — include reducing the recommended dose, allowing use of the drug up to 10 weeks of pregnancy (from seven weeks), approving a generic version and permitting it to be mailed (eliminating in-person doctor visits), among other measures.
Major pharmacy chains Walgreens and CVS announced this month they were certified to dispense the abortion drugs under the new rules.

Boxes of the drug mifepristone sit on a shelf at the West Alabama Women’s Center in Tuscaloosa, Ala., March 16, 2022. (Allen G. Breed, File)
Thirty-six states allow some form of access to mifepristone — 21 states allow full access and 15 allow restricted access, according to Fox News research. Fourteen states ban abortion completely, including medication abortion, except for a few exceptions.
Danco Laboratories, the drug’s manufacturer, had appealed to the Supreme Court seeking final review on the merits.
Dozens of advocacy groups, members of Congress and coalitions of states on both sides of the issue have filed legal briefs in recent weeks.
THE ARGUMENTS
The plaintiffs are led by four national medical associations of anti-abortion doctors linked to the conservative advocacy group Alliance Defending Freedom. They sued the FDA shortly after the Dobbs ruling, which ADF also spearheaded, hoping to build momentum on further abortion restrictions through litigation.
“The FDA recklessly removed its original safeguards like in-person doctor visits, leaving women to suffer serious complications alone,” said ADF Senior Counsel Erin Hawley, who will argue the case before the court. “It’s appalling that the FDA would eliminate even the initial in-person visit to check for life-threatening conditions like ectopic pregnancy based on studies it said were not adequate. Women deserve better.”
Lower courts agreed with anti-abortion groups, concluding the FDA did not fully consider the potential health risks to women when amending guidelines for mifepristone eight years ago.

People take part in a march for abortion rights from Pershing Square to City Hall in Los Angeles April 15, 2023. (Apu Gomes)
The Supreme Court will debate those questions and may revisit whether the original 24-year-old approval process for the drugs was similarly flawed under the federal Administrative Procedures Act.
Another key issue is whether those challenging the FDA authority have “standing” or capacity to sue, since those doctors allegedly have never actually prescribed mifepristone. They’ll also weigh whether they would suffer actual “harm” from the drug’s use since those plaintiffs are doctors, not patients.
“The stakes could not be higher for women across America,” said President Biden this month. “In the face of relentless attacks on reproductive freedom by Republican elected officials, Vice President Harris and I will continue to fight to ensure that women can get the health care they need, to defend the Food and Drug Administration’s independent and evidence-based approval and regulation of mifepristone and to restore the protections of Roe v. Wade in federal law.”
The Biden administration and abortion rights groups say continued access to mifepristone is vital. And they say a ruling limiting the FDA’s authority could have sweeping health care implications.
“If the logic of the lower court decision were allowed to stand, it would threaten to severely disrupt the pharmaceutical industry much more broadly, and it could prevent the FDA from doing the job that it’s supposed to be doing on behalf of the American people,” said Brianne Gorod, chief counsel at the progressive Constitutional Accountability Center.
“It would be a seismic shift in the way drugs are tested and brought to market, and so could really prevent individuals from getting life-saving medicines that they need.”

The Supreme Court (Mariam Zuhaib)
ONGOING CONTROVERSY
And this will not be the only abortion issue the Supreme Court is confronting.
The justices next month will hold oral arguments on a challenge to Idaho’s restrictions and whether they violate federal laws requiring hospitals to treat patients in life-threatening crises.
A federal court blocked enforcement of Idaho’s Defense of Life Act, which prohibits abortions unless necessary to save the life of the mother, on the grounds that the federal Emergency Medical Treatment and Labor Act preempts it. The state’s near-total abortion ban establishes criminal penalties for doctors who perform the procedure, except in narrow circumstances.
But the Biden administration counters that federal law requires emergency rooms to provide “stabilizing care,” including abortions, for a broader range of circumstances than a mother’s life, such as when a patient’s health is in “serious jeopardy.”
Other pending court challenges that may eventually reach the justices include whether the federal Title X family planning program can refer patients for abortions and whether those whose religious faith supports abortion access can file First Amendment constitutional challenges to state bans.
Pro-life women celebrate following the Supreme Court’s decision to overturn Roe v. Wade outside the Supreme Court in Washington June 24, 2022. (Gemunu Amarasinghe)
However, if the court rules on these disputes, the political impact could be immediate and seismic in the November election. Many progressives, while denouncing the 2022 Dobbs decision striking down the federal right to abortion, credited the controversy for energizing the base and swing voters. The midterm elections produced better-than-expected results for Democrats, while exposing a strategic vulnerability for Republicans and denying them an expected “red wave.”
CLICK HERE TO GET THE FOX NEWS APP
What the Supreme Court decides in June when confronted with the abortion pill case could have a similar impact, with nine appointed women and men in robes poised to reignite a divisive referendum on arguably the most controversial social issue facing the nation.
The mifepristone cases are FDA v. Alliance for Hippocratic Medicine (23-235) and Danco Laboratories, LLC v. Alliance for Hippocratic Medicine (23-236).