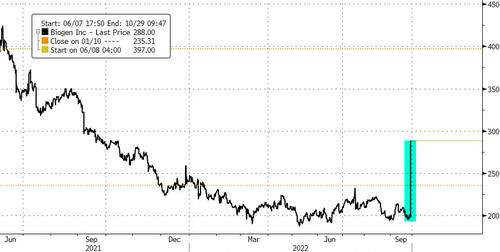
The pharmaceutical companies Biogen Inc. and Eisai Co. soared premarket after they announced positive results for a drug that slowed the progression of Alzheimer's disease.
The experimental drug, lecanemab, reduced the progress of the brain-wasting disease by 27% in an 18-month study involving 1,800 patients compared with a placebo, meeting the "primary endpoint" of the clinical trial "and key secondary endpoints with highly statistically significant results," the companies said in a statement.
Bloomberg noted some patients in the clinical trial experienced adverse side effects, such as brain swelling and bleeding, though severe cases were rare.
"Today's announcement gives patients and their families hope that lecanemab, if approved, can potentially slow the progression of Alzheimer's disease, and provide a clinically meaningful impact on cognition and function.
"Importantly, the study shows that removal of aggregated amyloid beta in the brain is associated with a slowing of disease in patients at the early stage of the disease. We want to thank the many patients who participated in this groundbreaking global study and want to acknowledge the clinical investigators who worked tirelessly to increase the enrollment of traditionally underrepresented populations. As pioneers in neuroscience, we believe defeating this disease will require multiple approaches and treatment options, and we look forward to continuing the discussion about the significance of these findings with the patient, scientific, and medical communities," Michel Vounatsos, Chief Executive Officer at Biogen, wrote in a statement.
Biogen's shares are up more than 45% premarket. Eisai closed up 17% in Tokyo. Eli Lilly & Co. and Roche Holding AG were also up premarket on the developments.
Here's what Wall Street analysts are saying about the lecanemab clinical trial (list courtesy of Bloomberg):
BMO Capital Markets (upgrades Biogen to outperform from market perform)
- The topline data is "as strong as can be," writes analyst Evan David Seigerman, and diminishes the possibility for CMS to deny broad coverage of the drug once approved
- Expects Lecanemab to win full FDA approval
Baird (upgrades Biogen to outperform from neutral)
- The reported study data is pretty much a best-case scenario, says analyst Brian Skorney
- This should not only lead to approval and reimbursement, but could make it challenging for competition to match
Barclays (equal weight-rated on Biogen)
- Lecanemab results were "clearly positive" and exceeded most Street expectations, and likely de-risks approval, writes analyst Carter Gould
- Provides Biogen with a path toward a return to growth against backdrop of a declining core business, and is a positive read- through to Eli Lilly's chances for donanemab
Raymond James (market perform-rated on Biogen)
- The study was a "clean win" and close to analyst Danielle Brill's best-case scenario
- Topline data looks to be supportive of full FDA approval and CMS coverage
- However, analyst is looking for more clarity on several variables
Cowen (outperform-rated on Biogen)
- The topline data appear to be a best-case scenario, writes analyst Phil Nadeau, adding it positions Lecanemab as a blockbuster
SVB Securities (outperform-rated on Biogen)
- Study results "clearly great news" for Biogen, writes analyst Marc Goodman, who expects the results to reinvigorate the Alzheimer's treatment space
The FDA granted priority review for lecanemab as a treatment for mild cognitive impairment due to Alzheimer's disease, and the regulator is expected to decide by Jan. 06.
The pharmaceutical companies Biogen Inc. and Eisai Co. soared premarket after they announced positive results for a drug that slowed the progression of Alzheimer’s disease.
The experimental drug, lecanemab, reduced the progress of the brain-wasting disease by 27% in an 18-month study involving 1,800 patients compared with a placebo, meeting the “primary endpoint” of the clinical trial “and key secondary endpoints with highly statistically significant results,” the companies said in a statement.
Bloomberg noted some patients in the clinical trial experienced adverse side effects, such as brain swelling and bleeding, though severe cases were rare.
“Today’s announcement gives patients and their families hope that lecanemab, if approved, can potentially slow the progression of Alzheimer’s disease, and provide a clinically meaningful impact on cognition and function.
“Importantly, the study shows that removal of aggregated amyloid beta in the brain is associated with a slowing of disease in patients at the early stage of the disease. We want to thank the many patients who participated in this groundbreaking global study and want to acknowledge the clinical investigators who worked tirelessly to increase the enrollment of traditionally underrepresented populations. As pioneers in neuroscience, we believe defeating this disease will require multiple approaches and treatment options, and we look forward to continuing the discussion about the significance of these findings with the patient, scientific, and medical communities,” Michel Vounatsos, Chief Executive Officer at Biogen, wrote in a statement.
Biogen’s shares are up more than 45% premarket. Eisai closed up 17% in Tokyo. Eli Lilly & Co. and Roche Holding AG were also up premarket on the developments.
Here’s what Wall Street analysts are saying about the lecanemab clinical trial (list courtesy of Bloomberg):
BMO Capital Markets (upgrades Biogen to outperform from market perform)
- The topline data is “as strong as can be,” writes analyst Evan David Seigerman, and diminishes the possibility for CMS to deny broad coverage of the drug once approved
- Expects Lecanemab to win full FDA approval
Baird (upgrades Biogen to outperform from neutral)
- The reported study data is pretty much a best-case scenario, says analyst Brian Skorney
- This should not only lead to approval and reimbursement, but could make it challenging for competition to match
Barclays (equal weight-rated on Biogen)
- Lecanemab results were “clearly positive” and exceeded most Street expectations, and likely de-risks approval, writes analyst Carter Gould
- Provides Biogen with a path toward a return to growth against backdrop of a declining core business, and is a positive read- through to Eli Lilly’s chances for donanemab
Raymond James (market perform-rated on Biogen)
- The study was a “clean win” and close to analyst Danielle Brill’s best-case scenario
- Topline data looks to be supportive of full FDA approval and CMS coverage
- However, analyst is looking for more clarity on several variables
Cowen (outperform-rated on Biogen)
- The topline data appear to be a best-case scenario, writes analyst Phil Nadeau, adding it positions Lecanemab as a blockbuster
SVB Securities (outperform-rated on Biogen)
- Study results “clearly great news” for Biogen, writes analyst Marc Goodman, who expects the results to reinvigorate the Alzheimer’s treatment space
The FDA granted priority review for lecanemab as a treatment for mild cognitive impairment due to Alzheimer’s disease, and the regulator is expected to decide by Jan. 06.







