
CVS and Walgreens will begin dispensing the abortion pill mifepristone in stores this month, only weeks before the Supreme Court is set to hear a case regarding the drug’s approval by the Food and Drug Administration.
The stores outlined to reporters on Friday their intention to begin dispensing mifepristone under the guidelines established by the FDA, slowly rolling out the controversial pills across states where it is legal for pharmacies to dispense the medication directly to patients.
“Walgreens has completed the FDA certification process to dispense mifepristone and expects to begin dispensing within a week, consistent with federal and state laws,” the company said in a press statement.
Walgreens spokesman Marty Maloney said that the chain would be providing pills within the next week to a handful of pharmacies in New York, Pennsylvania, Massachusetts, California, and Illinois. Maloney said the phased rollout is intended “to ensure quality, safety, and privacy for our patients, providers and team members.”
CVS spokeswoman Amy Thibault told the Washington Examiner that the company would begin its rollout “in the weeks ahead,” starting with Massachusetts and Rhode Island.
“We’re working with manufacturers and suppliers to secure the medication and are not yet dispensing it in any of our pharmacies,” Thibault said.
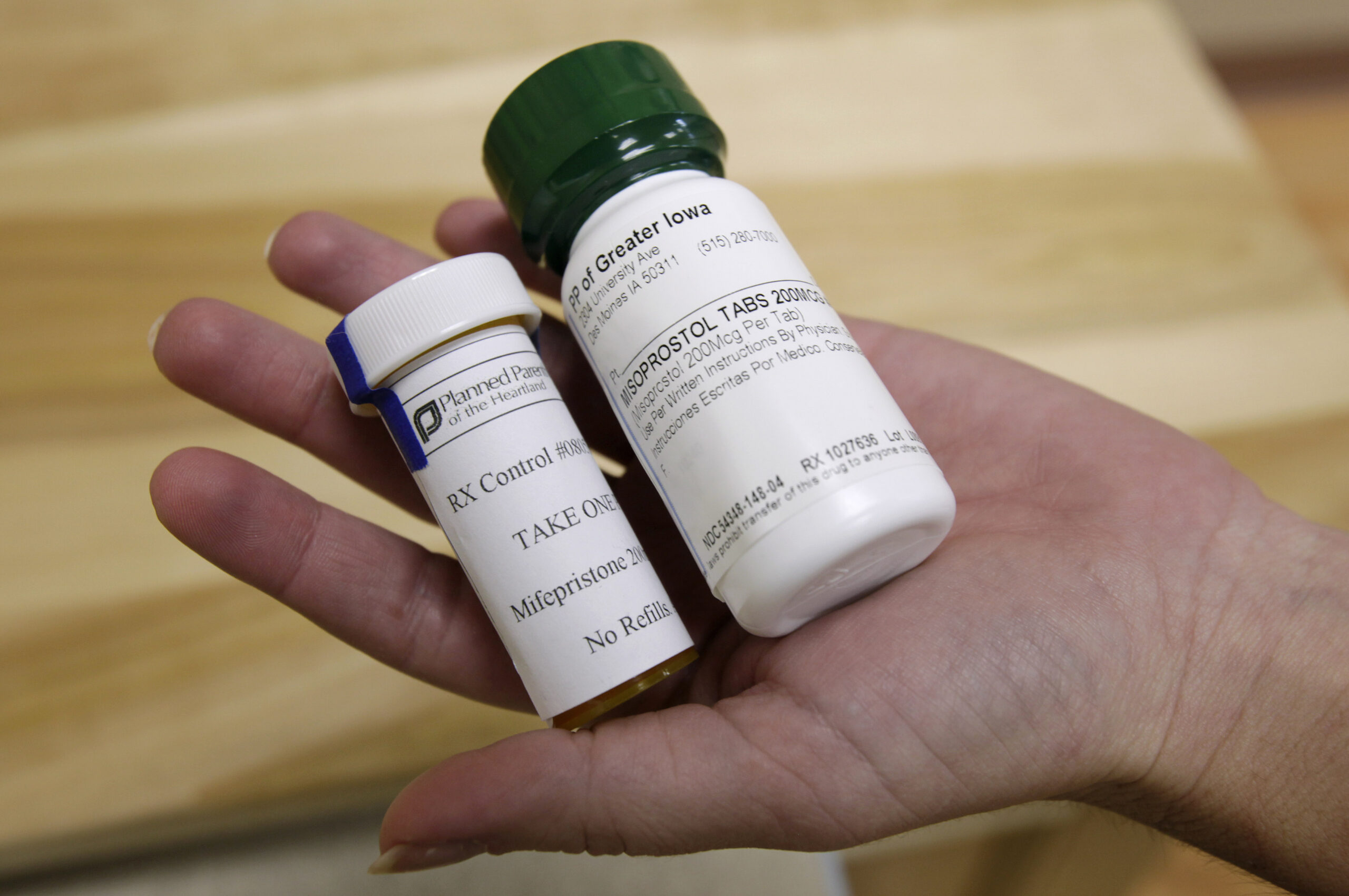
Mifepristone, the first agent in a two-pill chemical abortion process, works by blocking a woman’s progesterone receptors to stop fetal growth. The second pill in the series, misoprostol, is taken within 24 to 48 hours of mifepristone to begin uterine contractions to expel the fetus.
Mifepristone was originally approved by the FDA in 2000, but the agency loosened restrictions on the medication in 2016, allowing for the pill to be used up to 10 weeks gestation and with less monitoring from physician prescribers.
The FDA further relaxed restrictions on mifepristone during the COVID-19 pandemic to allow for patients to receive the medication without being physically examined by a healthcare provider, opening the door for by-mail access.
In 2023, Judge Matthew Kacsmaryk of the U.S. District Court for the Northern District of Texas ruled in favor of the Alliance for Hippocratic Medicine’s suit against the FDA’s original approval of mifepristone.
The panel of the 5th U.S. Circuit Court of Appeals rolled back part of the Texas judge’s decision, keeping the original FDA approval of the drug. The 5th Circuit did, however, restore regulations that effectively banned mail-order mifepristone access and required in-person doctor’s visits.
Danco Laboratories, the manufacturer of mifepristone, asked the high court in September to reverse the 5th Circuit’s decision, and the court agreed in December to review the case.
Oral arguments before the Supreme Court are scheduled for March 26. If the Supreme Court upholds the ruling of the 5th Circuit, patients may only be able to access mifepristone by visiting a clinic, doctor’s office, or pharmacy.
CVS is the largest pharmacy chain in the United States, with over 9,000 stores in all 50 states. Walgreens is the second-largest, with 8,500 stores in every state except North Dakota.
The Alliance Defending Freedom, representing the Alliance for Hippocratic Medicine, did not respond to the Washington Examiner’s request for comment.